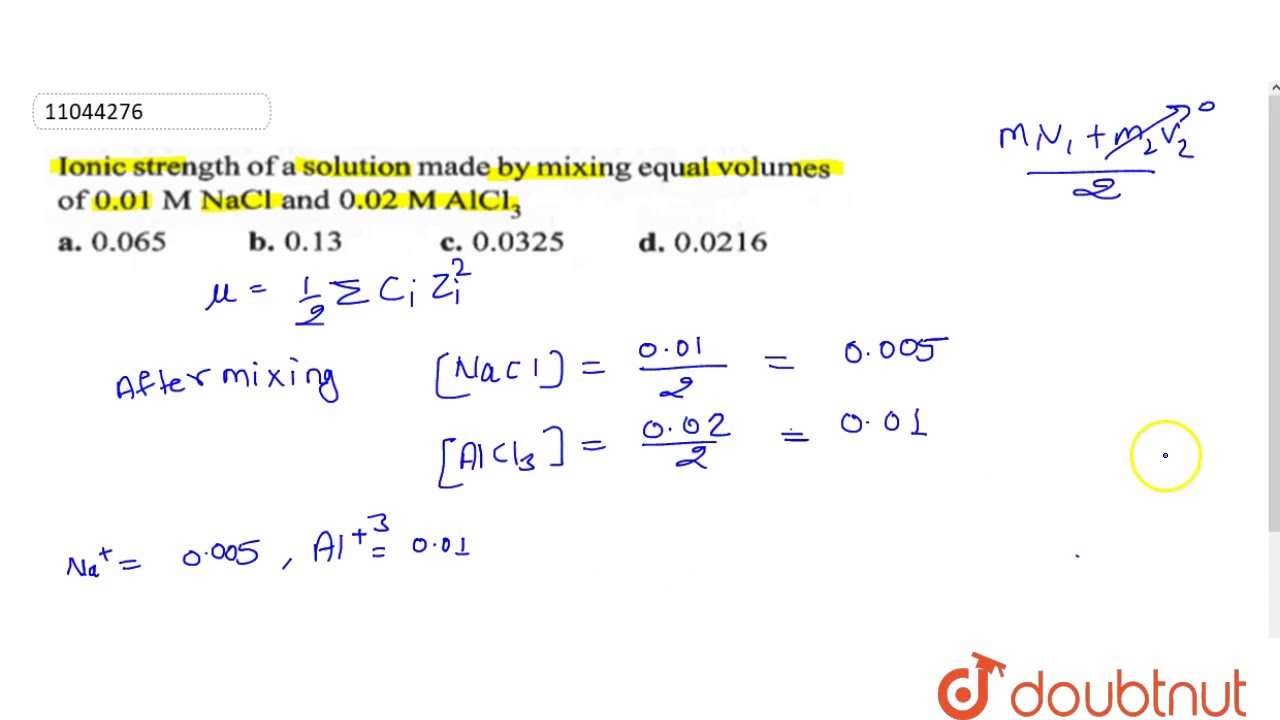
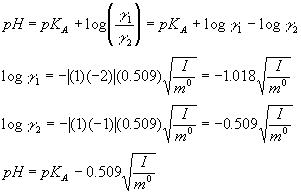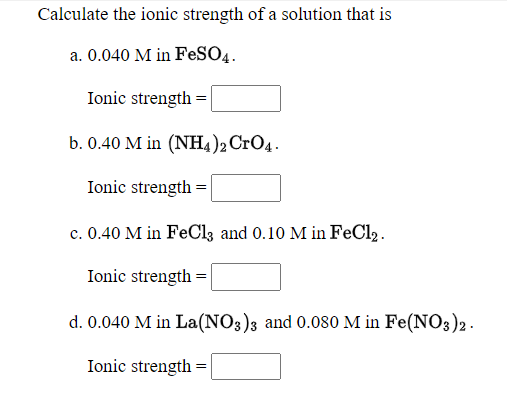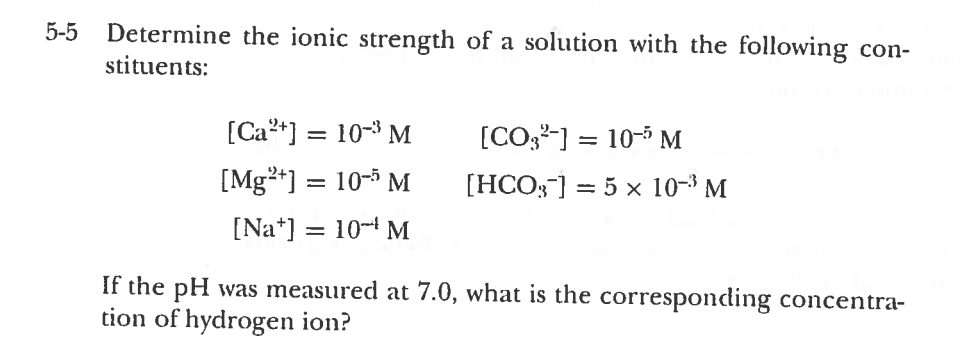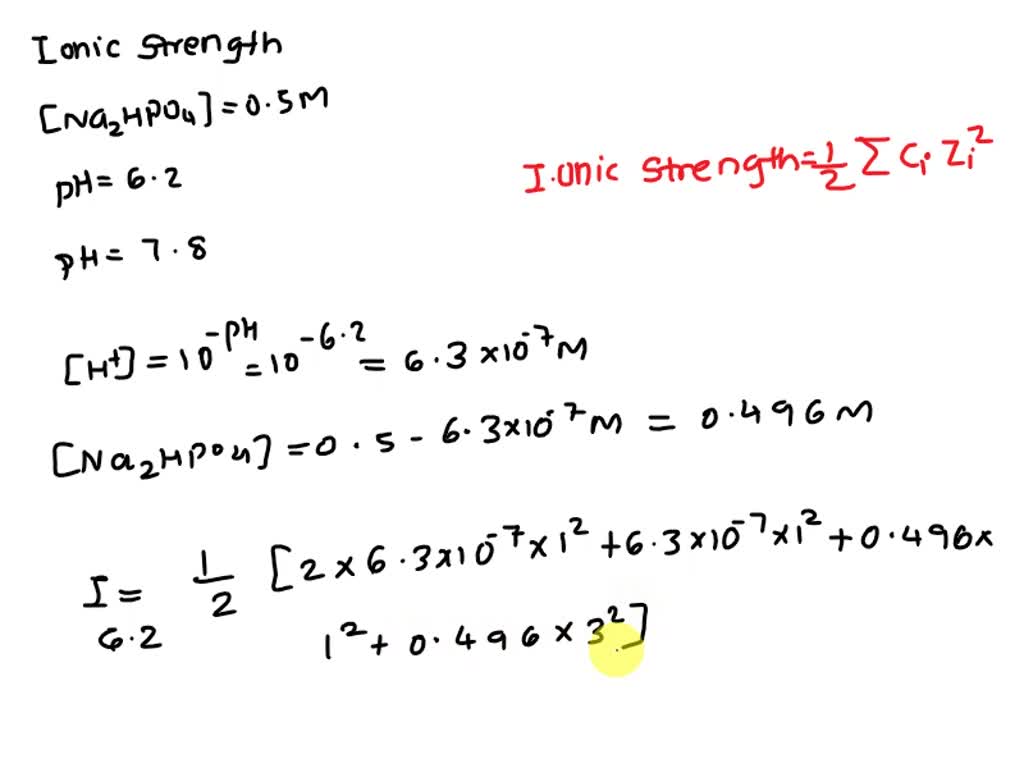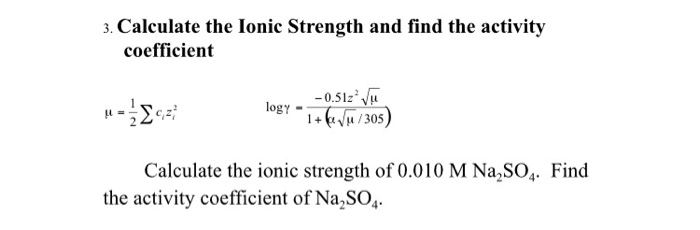Find the ionic strength of (Electrochemistry): i. 0.05 \ mol \ dm^{-3} \ KCl(aq) ii. 0.05 \ mol \ kg^{-1} LaFe(CN)_6(ag) Give detail explanation. (eq. why does CN have a charge of

Ionic strength - Introduction + total Explanation & formulas•MSc CHEMISTRY PHYSICAL• #notes - YouTube

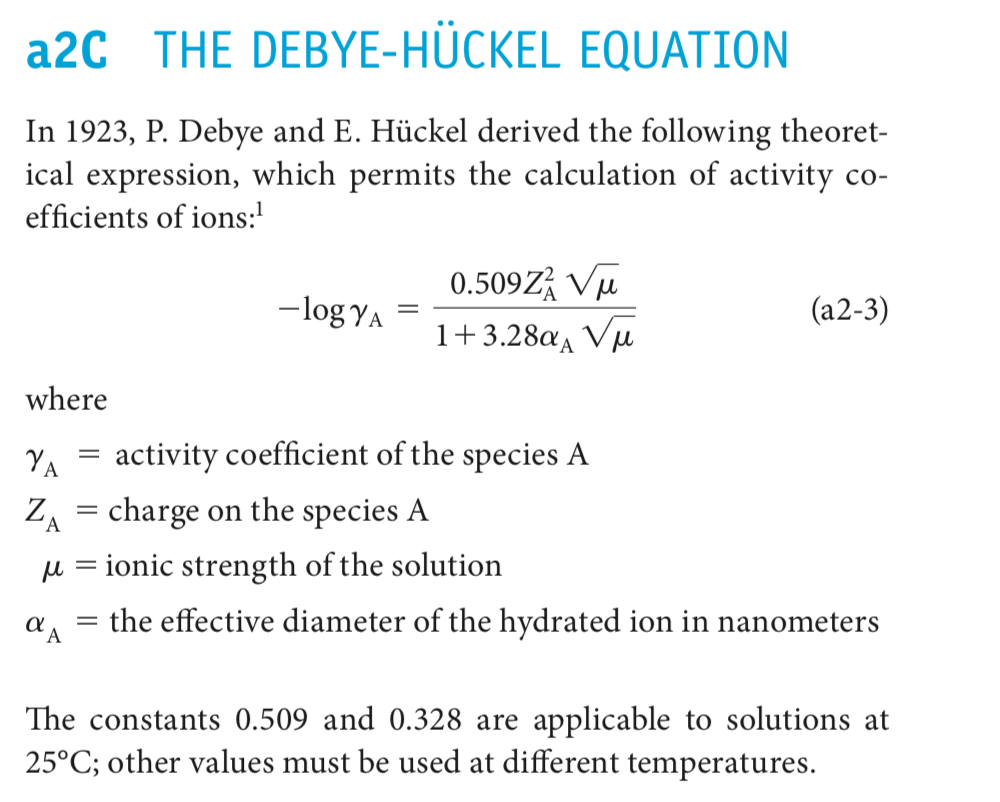
pH calculations and more in fundamentals of pharmaceutics. : What is ionic strength of solutions and how is it calculated?

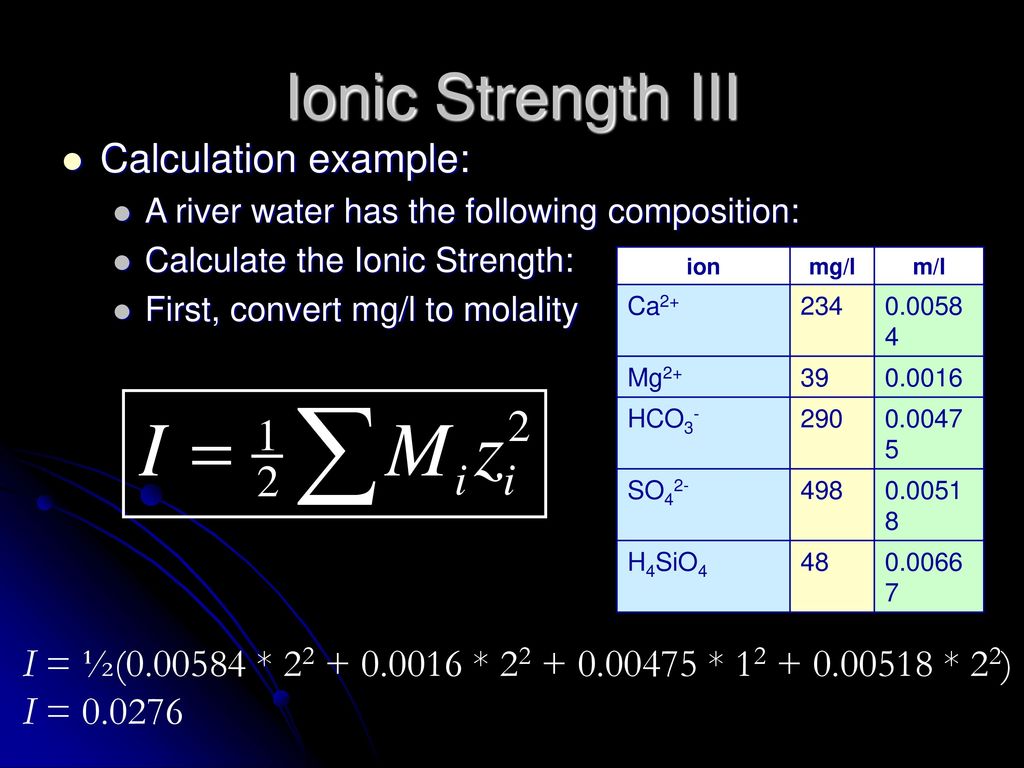
Ionic strength is sometimes stated as having units of molal (or molar) and other times stated as being unitless, depending on the book you read. The easiest. - ppt download

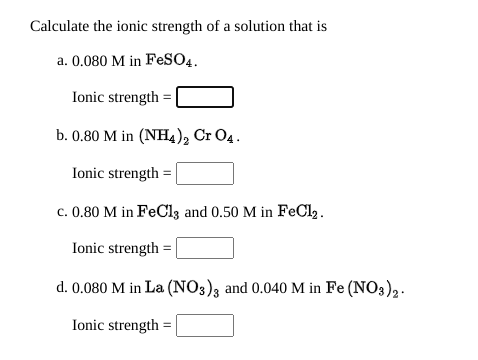
SOLVED: Calculate the ionic strength of a) a 0.2 M solution of MgCl2, and b) a 0.020 M NaCl plus 0.010 Na2SO4.
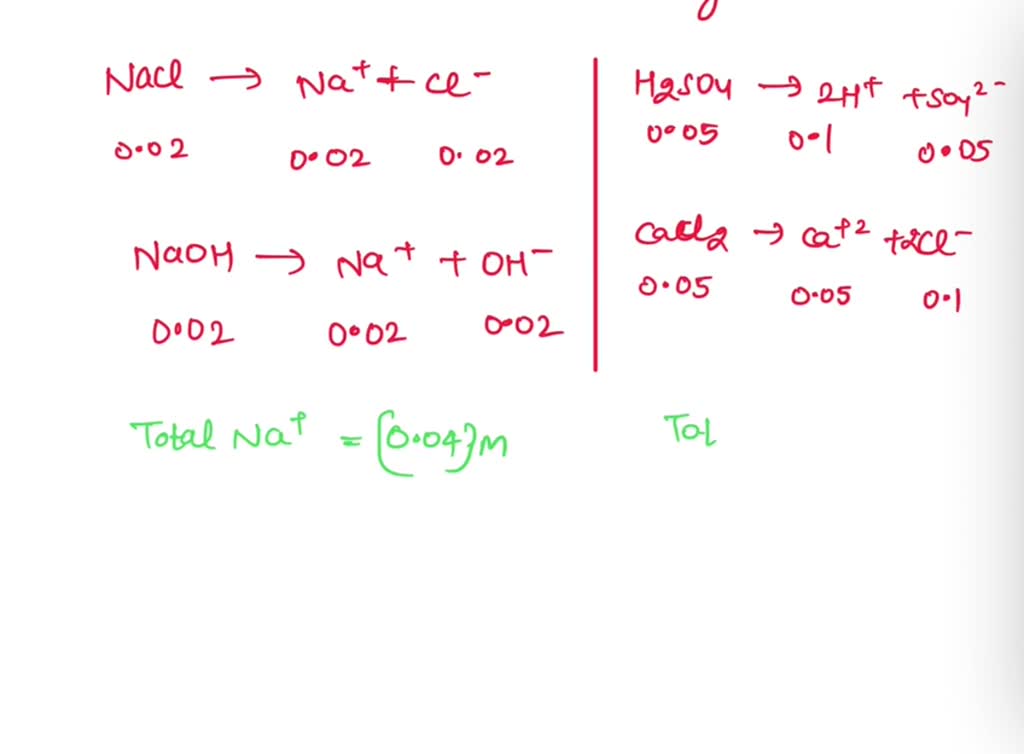
SOLVED: . Ionic strength: Calculate the activity of 0.02 M NaCl solution containing 0.02 M NaOH, 0.05 M H2SO4, 0.05 M CaCl2.

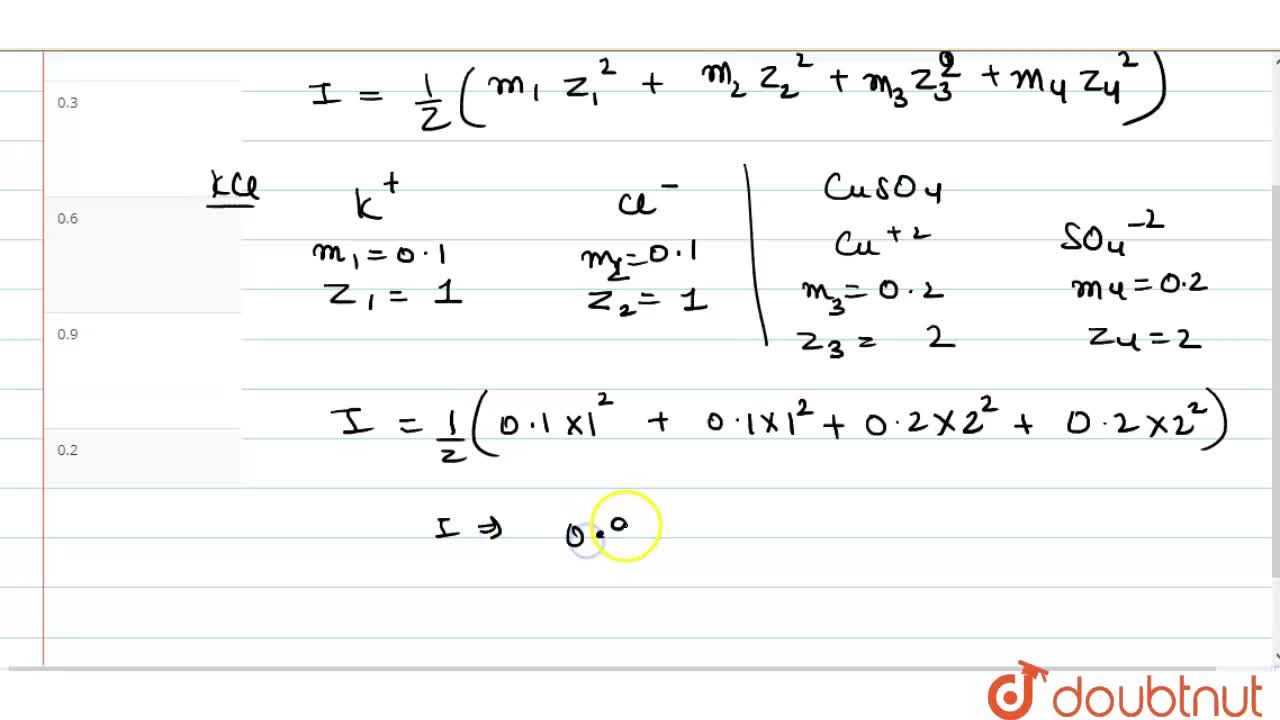
Calculation of Ionic strength||How to calculate ionic strength ||Rank booster-3||Cpet-2021|| - YouTube

25.4 g of iodine and 14.2 g of chlorine are made to react completely to yield a mixture of ICl and ICl3 . Calculate the number of moles of ICl and ICl3 formed.