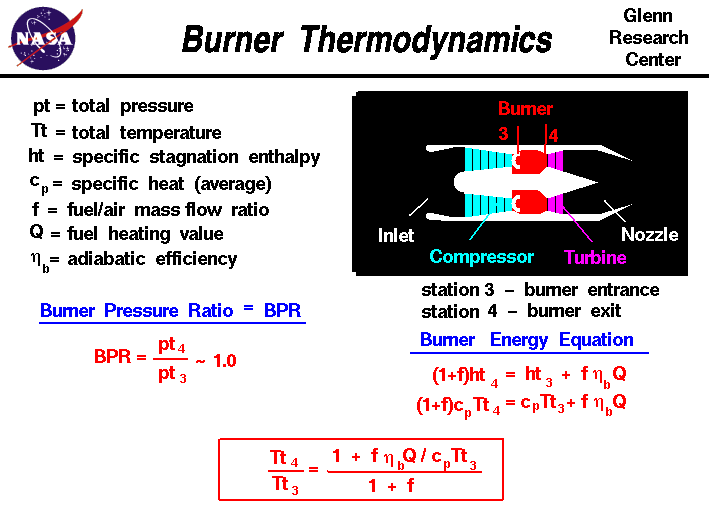
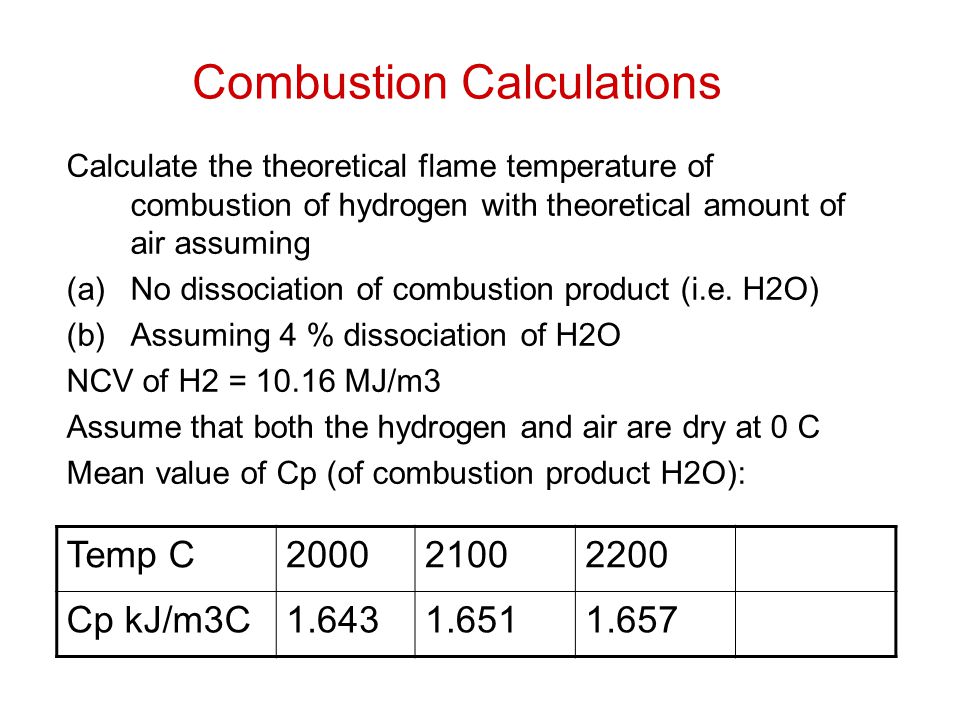
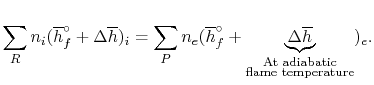Combustion Calculations Calculate the theoretical flame temperature of combustion of hydrogen with theoretical amount of air assuming (a)No dissociation. - ppt download

From the following data of heats of combustion, find the heat of formation of CH3OH(l) : CH3OH(l) + 32O2(g)⟶ CO2(g) + 2H2O(l);Δ H = - 726kJ C(s) + O2(g)⟶ CO2(g);Δ H = -

propulsion - How do you determine what the temperature will be in the combustion chamber of a rocket engine? - Space Exploration Stack Exchange